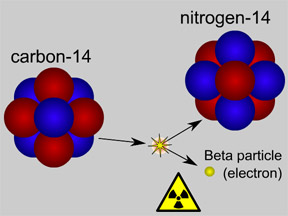
 Radioactivity is defined as the spontaneous disintegration
of certain atomic nuclei (e.g. carbon-14) into more stable forms (e.g. nitrogen-14). Radioactivity
half-life is a measure of how quickly this disintegration occurs, but not for
an individual atom.
Radioactivity is defined as the spontaneous disintegration
of certain atomic nuclei (e.g. carbon-14) into more stable forms (e.g. nitrogen-14). Radioactivity
half-life is a measure of how quickly this disintegration occurs, but not for
an individual atom.It is only measurable and meaningful when considering a large number of radioactive atoms. Then one can talk about how long it takes for half the atoms to decay (i.e. for their nuclei to disintegrate); but looking at one single atom it is absolutely impossible to know whether its nucleus will have decayed in 1 second or 1000 years. One can only talk about the most probable lifetime.
For instance, radium 226 decays into polonium, lead, bismuth
and other elements with a half-life of 1601 years. Carbon-14 decays into
nitrogen with a half-life of 5,740 years. Yet these figures say nothing about
any one atom of Ra-226 or C-14. They are statistical in nature.
Until recently it was assumed that the half-life of samples
of elements were constants of nature. Nothing could change them. They reflected
the quantum phenomena that go on inside the nucleus of an atom.
It is now looking as though this 80 year old assumption is
wrong. There is growing evidence that the radioactivity half life varies with
processes occurring inside the sun and the position of the earth in its orbit.
This has been found independently in at least three different labs:
Experiments need to be carried out on carbon-14 because this
is used extensively in radiocarbon dating. Variations of a fraction of a
percent would not seriously affect existing carbon dating data on archaeological
or palaeontological samples. Yet we know that the sun’s activity has not been
constant. It is thought to be 20 – 25 % more active now than at the time of the
earth’s formation. If miniscule variations in solar activity affect
radioactivity very slightly, what would be the effect of large variations? Perhaps none. But if not, the implications for history could be enormous.
Even the small effects discovered to date are inexplicable
in terms of existing elementary particle physics. E.g. a new kind of neutrino,
the neutrello, has been proposed to explain them but there is no evidence that
such a particle exists. It may even be necessary to hypothesise a fifth force in
addition to the four already known: electromagnetic, weak nuclear, strong
nuclear and gravitational.
Another case of interesting
times ahead for physics and possibly for history.
John
Author 2077 AD
See, for example,
proton size puzzle